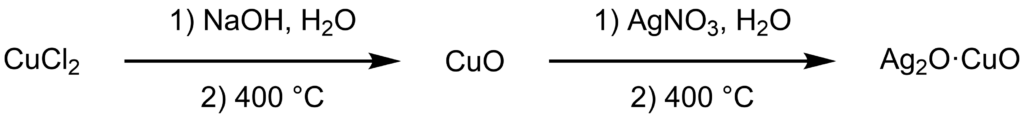
In my quest for modifications of the seemingly new azo dye I have made before, I want to use vanillyl alcohol and vanillic acid as coupling partners for diazotized 5-amino-2-naphthalenesulfonic acid. While the synthesis of vanillyl alcohol from vanillin is straight forward, making vanillic acid is a bit more tricky. Two methods have been published in the highly reputable journal OrgSyn, however one method requires stoichiometric amounts of expensive silver oxide, and the other involves melting an eutectic of potassium- and sodium hydroxide which is overall a very hazardous procedure. An alternative approach was published by Q. Tian et al., and while the unfortunate stereotypes about the quality of chinese chemistry publications keep reinforcing themselves, the provided reaction conditions and data seemed reasonable enough to give it a try. Their procedure involves the preparation of a mixed copper/silver oxide catalyst, which supposedly allows for the air oxidation of benzaldehydes at 70 °C. The oxidation of aldehydes in presence of copper is a well known phenomenonbased on a two step process. First, copper(II) oxide undergoes a redox reaction with the aldehyde to form copper(I) oxide and the corresponding acid. The copper(I) oxide is then regenerated by autoxidation. In this post I describe my attempt at making the described catalyst, and in a followup post I will report on the results of the aldehyde oxidation. The authors of the cited paper used analytical grade copper(II) oxide as starting material, but since I did not have any i had to make it first by thermal decomposition of freshly prepared copper(II) hydroxide.
| Substance | M [g/mol] | n [mmol] | m [g] | V [ml] | Eq. |
|---|---|---|---|---|---|
| Copper(II) chloride dihydrate | 170.48 | 29.33 | 5.00 | – | 1.0 |
| Sodium hydroxide | 40.00 | 87.99 | 3.52 | – | 3.0 |
| Silver nitrate | 169.87 | 1.00 | 0.17 | – | 0.03 |
Experimental:
1) Copper(II) oxide:
In a tall 250 ml beaker, copper(II) chloride dihydrate (5.00 g, 29.33 mmol, 1.0 eq.) was dissolved in water (50 ml). A solution of sodium hydroxide (3.52 g, 87.99 mmol, 3.0 eq.) in water (25 ml) was prepared and immediately added to the copper(II) chloride solution with strong stirring. The thus formed precipitate of copper(II) hydroxide was filtered off and washed with 50 ml portions of water until the filtrate was neutral (8 washings were necessary in this case). The precipitate was transferred to a 50 ml glazed silica crucible and heated to 360 °C at a rate of less than 10 °C per minute. After 30 minutes had elapsed at the target temperature, the resulting brittle chunks were allowed to cool down and broken up with a mortar and pestle yielding copper(II) oxide (2.15 g, 27.03 mmol, 92%) as a compact dark brown powder.
2) Mixed oxide catalyst
In a 25 ml beaker, silver nitrate (0.17 g, 1.00 mmol, 0.03 eq.) was dissolved in water (7 ml), and the previously prepared copper(II) oxide (2.15 g, 27.03 mmol, 1.0 eq.) was added. A suspension was formed by stirring the mixture with a spatula for 5 minutes, and the water was removed by heating the slurry to 80 °C for two hours. The resulting solid was broken up into coarse chunks and calcined at 400 °C for one hour (10 °C per hour heating rate). After cooling to room temperature, the solid was pulverized with a mortar and pestle to give the mixed metal oxide catalyst (2.23 g, 98%) as a fine dark gray powder.
Adapted from: Q. Tian et al., Molecules 2008, 13(4), 948-957






