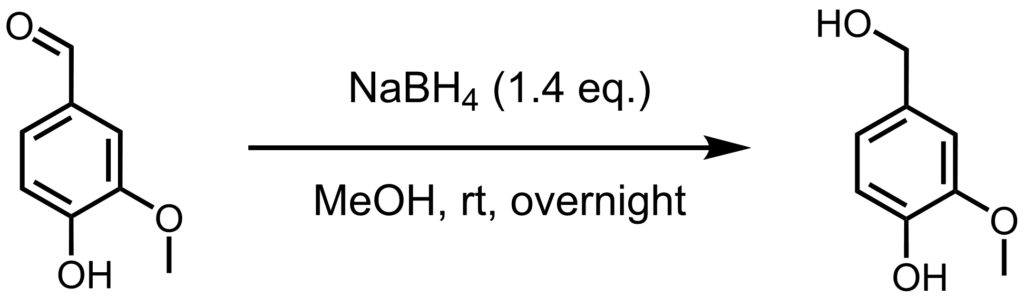
In my previous experiment, I synthesized an azo dye. While that worked well and the dye gave some pretty colors depending on the pH, it does not seem to be stable under moderately acidic conditions. I suspect that the aldehyde group of the vanillin substructure is the culprit, and while there are several ways to prevent this, it is a good idea to first confirm this suspicion. To do this, one may simply convert the aldehyde to a more stable group, in this case to an alcohol. I do plan to also test vanillic acid and modifications other than reductions and oxidations, but conversion to vanillic alcohol is the most straight forward approach.
| Substance | M [g/mol] | n [mmol] | m [g] | V [ml] | Eq. |
|---|---|---|---|---|---|
| Vanillin | 152.15 | 6.57 | 1.00 | – | 1.0 |
| Sodium borohydride | 37.83 | 9.36 | 0.30 | – | 1.4 |
| Methanol | 32.04 | – | – | 50 | – |
Experimental:
In a 100 ml round bottom flask, vanillin (1.00 g, 6.57 mmol, 1.0 eq.) was dissolved in methanol (50 ml). To this solution was added sodium borohydride (0.30 g, 9.36 mmol, 1.4 eq.) in small portions and with strong stirring. After the addition was complete, a drying tube filled with silica gel was attached, and the mixture was stirred overnight. The solvent was removed in vacuo, and water (50 ml) was added, initially forming a suspension which dissolved after stirring for 15 minutes. The solution was neutralized (pH ~ 7) with solid ammonium chloride and extracted seven times with diethyl ether (20 ml). The combined organic phases were dried over sodium sulfate, and the solvent was removed in vacuo yielding vanillyl alcohol (0.82 g, 5.39 mmol, 82%) as a crystalline white solid.
Adapted from: V. I. Vinogradova et al., Chem. Nat. Comp., 26(1), 54, 1990
Additional info about the product of this synthesis can be found on its info page.


