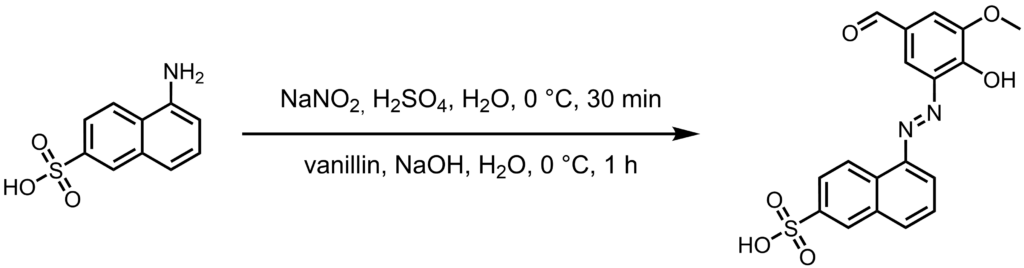
Azo compounds are one of the most versatile class of dyes. Their discovery changed history, making some people incredibly rich and some others poor in return. intriguing properties are the ease of synthesizing them as well as the vast range of reagent combinations and therefore range of colours attainable. The high versatility of viable starting materials is also the reason why today, decades after their discovery, new azo dyes are still published. As for the dye described here, I was unable to find any literature references referencing its synthesis or properties. This is really a shame, since the pH dependent color range is quite pretty. Now, as mentioned in the warning notice, azo dyes tend to be mildly carcinogenic, so care has to be taken when handling them. For this reason I opted to only conduct this experiment on a 100 mg scale, but since azo dyes are typically very potent, this was not an issue.
| Substance | M [g/mol] | n [mmol] | m [g] | V [ml] | Eq. |
|---|---|---|---|---|---|
| 5-Aminonaphthalene- 2-sulfonic acid | 223,25 | 0.448 | 0.100 | – | 1.0 |
| Sulfuric acid (15 wt%) | 98,08 | 1.340 | – | 0.8 | 3.0 |
| Sodium nitrite | 69,00 | 0.672 | 0.046 | – | 1.5 |
| Vanillin | 152,15 | 0.448 | 0.068 | – | 1.0 |
| Sodium hydroxide (1.0 M in water) | 40,00 | 2.700 | – | 2.7 | 6.0 |
Experimental:
In a 10 ml round bottom flask, 5-Aminonaphthalene-2-sulfonic acid (0.100 g, 0.448 mmol, 1.0 eq.) was suspended in water (2.0 ml) and sulfuric acid (15wt%, 0.8 ml, 1.340 mmol, 3.0 eq.) by stirring strongly for 30 minutes. The suspension was cooled to 0 °C with an ice bath, and a solution of sodium nitrite (0.046 g, 0.672 mmol, 1.5 eq.) in water (1.0 ml) was added dropwise over the course of ten minutes. After stirring for another 20 minutes, the resulting beige suspension was transferred dropwise to a 25 ml beaker containing an ice cold solution of vanillin (0.068 g, 0.448 mmol, 1.0 eq.) in 1.0 M aqueous sodium hydroxide (2.7 ml, 2.700 mmol, 6.0 eq.). The beaker was swirled for the duration of the addition, and the solution was allowed to warm to room temperature over the course of an hour. The crude reaction mixture was nearly black, but adding a drop of the solution to a piece of tissue paper revealed that the color of the solution was in fact orange. Addition of sulfuric acid resulted in a pink coloration.
Adapted from: Becker, H. G., Berger, W. & Domschke, G. (1998). Organikum: Organisch chemisches Grundpraktikum (20. Aufl.). Wiley-VCH.
Analytical data and further information can be found in the info sheet of this compound.




